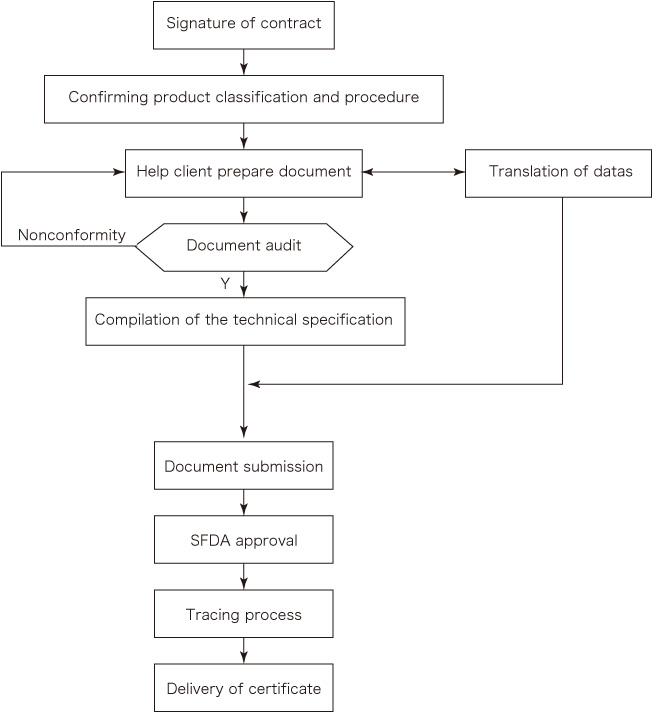
CFDA Medical Device Registration
Testing of Medical Device
Clinical Trial of Medical Device
Registration of Class I Import Medical Device
Docs required for registration of Class I import medical device:
(1) The certificate of the legal production qualification of the Manufacturer.
(2) The qualification certificate of the applicant
(3) The certificate recognized or approved by the government of the Country (Region) of Origin to authorize the products as medical devices to enter into the market of the country.
(4) The Standards of the Products to be Registered shall apply the Provisions for the Management of the Medical Devices Standards
(5) Operation Manual of the Products
(6) The Product Quality Guaranty presented by the Manufacturer, to promise that the quality of the products registered and sold in China are unanimously the same as that of the identical products put into market in the Country (Region) of Origin.
(7) The certificate of commission for the After-Sale Service Agency designated in China, the letter of commitment and business certificate of the commissioned agency.
(8) The Self-Guarantee Declaration on the authenticity of the materials submitted.
We will provide the template for docs mentioned aboves.
Docs required for registration of Class I import medical device:
(1) The certificate of the legal production qualification of the Manufacturer.
(2) The qualification certificate of the applicant
(3) The certificate recognized or approved by the government of the Country (Region) of Origin to authorize the products as medical devices to enter into the market of the country.
(4) The Standards of the Products to be Registered shall apply the Provisions for the Management of the Medical Devices Standards
Docs required for registration of Class I import medical device:
(1) The certificate of the legal production qualification of the Manufacturer.
(2) The qualification certificate of the applicant
(3) The certificate recognized or approved by the government of the Country (Region) of Origin to authorize the products as medical devices to enter into the market of the country.